RACE-V
-
Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodeling, and Vascular Destabilisation in the Progression of AF (RACE V)
RACE-V is a network of researchers from Groningen, Maastricht and Leiden, financed by the Dutch Heart Foundation. Together, we are investigating how AF affects coagulation factors both in the blood and in atrial tissue and how these factors in turn alter the atrial tissue and lead to progression of AF.
Atrial fibrillation (AF) is not benign. It commonly progresses from paroxysmal self-terminating to persistent non-selfterminating AF. AF frequently starts unnoticed, which allows it to progress freely and cause adverse events before it is detected. Current diagnostic and treatment modalities focus on AF in later stages. This is the most important reason why AF interventions are ineffective in reducing adverse events.
Our central hypothesis links AF progression to vascular risks via hypercoagulability, i.e. activation of blood coagulation through thrombin. Hypercoagulability is an obvious, but largely unexplored, disease mechanism in AF with its well-known predisposition for stroke. Based on preliminary results, we consider this an important link between early AF and thrombin-induced AF progression mediated though atrial vascular remodelling and fibrosis. In our basic research we will study how AF affects blood coagulation and vascular function, how hyper¬coagulability and vascular alterations enhance atrial remodelling and AF, how common genetic variants affect atrial gene expression, and how they modify the effect of mechanisms on AF progression.
Translation to patients will be implemented in a registry of patients with early selfterminating AF. Using continuous rhythm monitoring combined with in-depth molecular-to-clinical phenotyping at the time of calamity (AF attack) and outside the time of calamity (sinus rhythm) including blood biomarkers of hypercoagulability, vascular disease, and fibrosis as well as electrocardiographic and imaging biomarkers, we will assess determinants of AF progression. In this way we aim to produce new diagnostic algorithms, electrical and biochemical biomarkers, and novel therapeutic modalities to prevent AF progression and its complications.
-
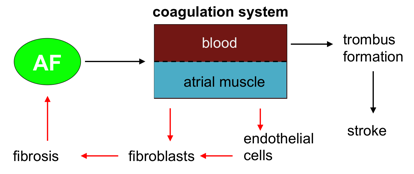
Figure - It is well known that atrial fibrillation (AF) activates the blood coagulation system, leading to trombus formation in the atria that increases the risk for stroke (black arrows). In the RACE-V network, we will study the activity of coagulation factors within the atrial wall, and how these affect myocytes, fibroblasts and endothelial cells, leading to fibrosis and perpetuating AF (red arrows).
